The world of biotechnology is advancing at an incredible pace, and at the center of this revolution is CRISPR-Cas gene editing for the treatment of diseases. This groundbreaking tool enables scientists to make precise, targeted modifications in the DNA of living organisms, offering hope for curing genetic disorders, cancers, and infectious diseases.
Unlike older methods of genetic engineering, CRISPR-Cas provides accuracy, speed, and cost-efficiency. It opens the door to a new era of precision medicine where treatments are tailored to the genetic blueprint of each patient.
What Is CRISPR-Cas Gene Editing for the Treatment of Diseases?
CRISPR-Cas gene editing for the treatment of diseases is a biotechnology technique that allows researchers to cut, remove, or replace DNA segments with unparalleled precision.
Key features include:
- CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats): DNA sequences that guide editing.
- Cas proteins (e.g., Cas9): Molecular scissors that cut the DNA at specific points.
- Guide RNA: Directs Cas proteins to the target sequence.
- Repair mechanisms: The cell’s natural repair system inserts or replaces DNA fragments.
This method is powerful, versatile, and adaptable across multiple disease areas.
Why Is CRISPR-Cas Gene Editing for the Treatment of Diseases Important?
The importance of CRISPR-Cas gene editing for the treatment of diseases lies in its potential to revolutionize healthcare:
- Cures genetic disorders: Addresses conditions like sickle cell anemia and muscular dystrophy.
- Cancer therapy: Targets mutations at the genetic level.
- Infectious disease control: Provides new methods to fight viruses like HIV.
- Precision medicine: Enables highly personalized treatments.
- Cost efficiency: Makes gene therapy more accessible.
This tool doesn’t just treat symptoms—it corrects the root causes of diseases.
How Does CRISPR-Cas Gene Editing for the Treatment of Diseases Work?
The process involves a series of steps:
- Step 1: Designing guide RNA to match the DNA sequence linked to the disease.
- Step 2: Introducing Cas proteins into the cell along with the guide RNA.
- Step 3: Cutting the DNA at the exact point of mutation.
- Step 4: Repairing or replacing the DNA using templates or natural repair systems.
- Step 5: Monitoring results to ensure accuracy and minimize side effects.
This precise workflow underpins the success of CRISPR-Cas gene editing for the treatment of diseases.
What Are the Benefits of CRISPR-Cas Gene Editing for the Treatment of Diseases?
The benefits span across healthcare and research:
- Precision: Targets specific genes without affecting surrounding DNA.
- Versatility: Applicable to many diseases and organisms.
- Speed: Accelerates research and clinical trials.
- Affordability: Less expensive than older gene-editing tools.
- Long-term potential: Offers permanent cures instead of temporary treatments.
For patients, it brings hope; for science, it offers a powerful instrument.
What Are the Applications of CRISPR-Cas Gene Editing for the Treatment of Diseases?
Applications are diverse and groundbreaking:
- Genetic disorders: Correcting mutations in cystic fibrosis, hemophilia, and Huntington’s disease.
- Cancer treatment: Engineering immune cells to attack tumors.
- Infectious diseases: Editing genes to block viral replication.
- Blood diseases: Transforming therapies for sickle cell anemia and beta-thalassemia.
- Drug discovery: Creating accurate disease models for testing.
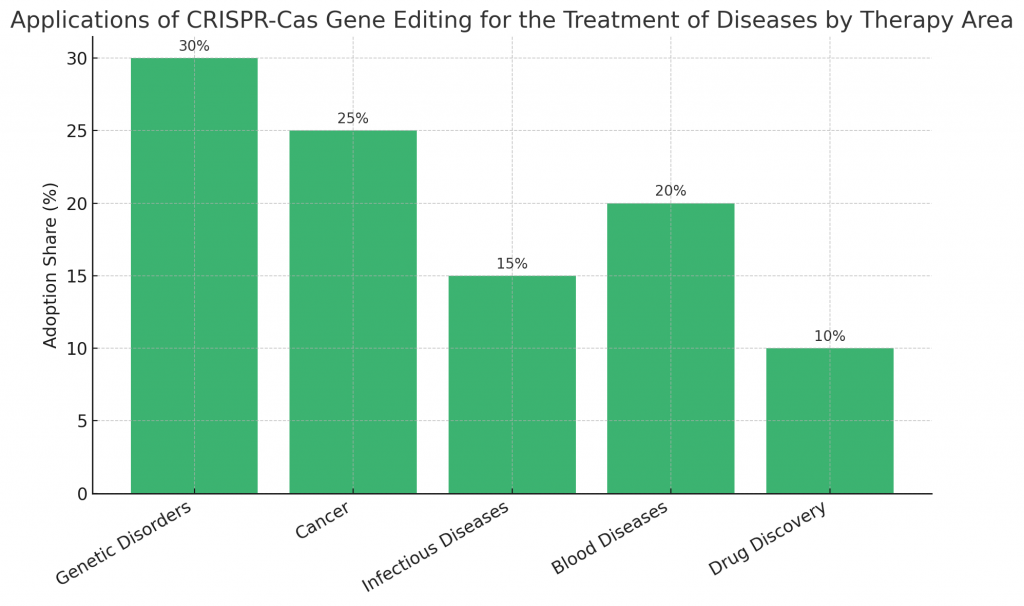
These applications highlight its potential as a universal medical tool.
Which Companies Are Leading in CRISPR-Cas Gene Editing for the Treatment of Diseases?
Major corporations are investing heavily:
- CRISPR Therapeutics: Focus on genetic blood disorders.
- Editas Medicine: Clinical trials for rare diseases.
- Intellia Therapeutics: Leading in in-vivo gene editing.
- Verve Therapeutics: Tackling cardiovascular disease.
- Beam Therapeutics: Advancing base editing innovations.
These pioneers are commercializing CRISPR-Cas gene editing for the treatment of diseases.
Which Startups Are Innovating Rapidly?
Startups are bringing agility and new concepts:
- Caribou Biosciences: Foundational CRISPR innovations.
- Mammoth Biosciences: Expanding Cas protein applications.
- Scribe Therapeutics: Engineering next-generation CRISPR tools.
- eGenesis: Applying CRISPR to organ transplantation.
- SNIPR Biome: Editing microbes to fight infections.
They represent the next wave of innovation.
What Do Patents and TRL Levels Indicate?
Patent activity reveals focus on:
- Cas protein engineering.
- Delivery mechanisms for in-vivo editing.
- Therapeutic applications in oncology and neurology.
- Ethical and regulatory frameworks.
Technology Readiness Levels (TRLs):
- Blood disorder treatments: TRL 8–9, in advanced clinical stages.
- Cancer therapies: TRL 6–8, progressing in trials.
- Infectious disease applications: TRL 5–7, under development.
- Organ editing and xenotransplantation: TRL 4–6, experimental stage.
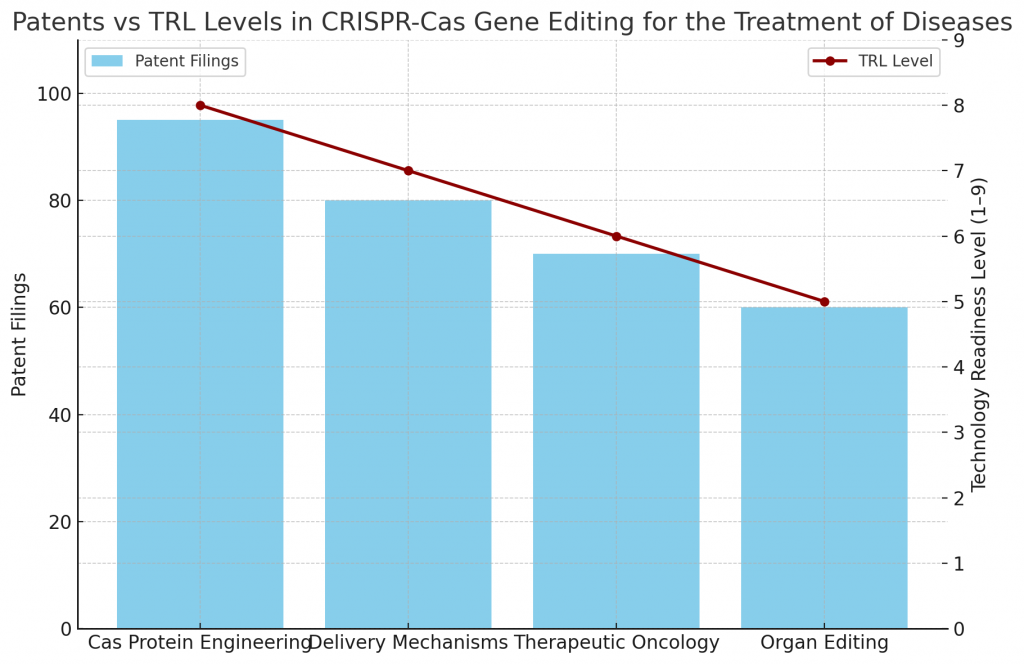
This demonstrates both maturity and untapped potential.
What Are the Challenges in Adoption?
Despite its promise, challenges exist:
- Ethical concerns: Germline editing remains controversial.
- Off-target effects: Risk of unintended mutations.
- Delivery methods: Ensuring safe and efficient delivery into cells.
- Regulatory hurdles: Strict oversight required for medical use.
- Cost of therapies: Needs further reduction for mass adoption.
Overcoming these challenges will define the success of CRISPR-Cas gene editing for the treatment of diseases.
What Is the Future Outlook?
The outlook is highly promising:
- Short term (1–5 years): More clinical trial approvals for genetic disorders.
- Medium term (5–10 years): Expansion into cancer and cardiovascular treatments.
- Long term (10+ years): Integration into mainstream healthcare for multiple conditions.
This confirms that CRISPR-Cas gene editing for the treatment of diseases will be a cornerstone of 21st-century medicine.
How Can PatentsKart Help?
PatentsKart empowers innovators in CRISPR-Cas gene editing for the treatment of diseases with:
- Patent landscaping to identify opportunities.
- Competitor analysis to monitor global leaders.
- Freedom-to-operate studies to reduce risks.
- TRL benchmarking to assess maturity.
- Commercialization strategies to accelerate adoption.
This support ensures researchers and companies stay competitive.
Conclusion
The promise of gene editing is no longer confined to science fiction. CRISPR-Cas gene editing for the treatment of diseases is unlocking cures once thought impossible, offering new hope for millions.
By correcting genetic errors at their source, this technology is not just treating diseases—it is reshaping medicine itself.
FAQs About CRISPR-Cas Gene Editing for the Treatment of Diseases
Q1. What is CRISPR-Cas gene editing for the treatment of diseases?
It is a biotechnology method that precisely modifies DNA to cure genetic disorders and other illnesses.
Q2. Why is it important?
It targets root causes of diseases, enabling permanent cures and precision medicine.
Q3. Which companies are leaders?
CRISPR Therapeutics, Editas Medicine, Intellia, Verve, and Beam Therapeutics.
Q4. What challenges exist?
Ethical debates, off-target risks, delivery methods, and regulatory frameworks.
Q5. How can PatentsKart help innovators?
By offering IP analysis, TRL studies, and commercialization support.







