What is non-invasive EKG and insulin monitoring and why does it matter?
Non-invasive EKG and insulin monitoring is reshaping how we track heart and metabolic health. The idea is simple: continuous, painless sensing of cardiac electrical activity and glucose levels without finger pricks or hospital-grade electrodes. For people with diabetes or heart conditions, this means faster decisions, fewer clinic visits, and a clearer picture of daily physiology.
Short, continuous data beats episodic snapshots. When devices report trends instead of single points, clinicians and patients can spot early warning signs and act before complications occur.
How does non-invasive EKG and insulin monitoring work in simple terms?
Most modern systems combine several sensing methods and smart analytics. Typical components include:
- Optical sensors (PPG, NIR) on the wrist or patch.
- Dry electrodes or textile electrodes embedded in clothing for ECG-like signals.
- Bioimpedance to infer cardiovascular changes.
- Raman or spectroscopy techniques for glucose estimation.
- Microfluidic sampling or sweat sensors for chemically derived values.
- Edge-AI filters motion artifacts and fuses signals, sending cleaned metrics to apps or cloud dashboards.
The sensor stack collects raw signals; the software converts them into clinically relevant metrics (rhythm, glucose trend, alerts). That fusion—hardware plus algorithms—is where accuracy is won or lost.
What technologies power non-invasive EKG and insulin monitoring?
Key technology building blocks:
- Dry electrodes and textile integration for unobtrusive cardiac sensing.
- Photoplethysmography (PPG) for heart-rate and rhythm estimation.
- Bioimpedance cardiography for stroke and circulation proxies.
- Raman and near-infrared (NIR) spectroscopy for optical glucose estimation.
- Microfluidic patches that access interstitial fluid or sweat without lancets.
- Edge computing and federated learning to protect privacy while improving models.
Each method has trade-offs—optical methods are sensitive to motion and skin tone; spectroscopy needs robust calibration; microfluidics must manage sample variability. Hybrid solutions reduce single-method weaknesses.
Who are the major companies leading this space and what are they doing?
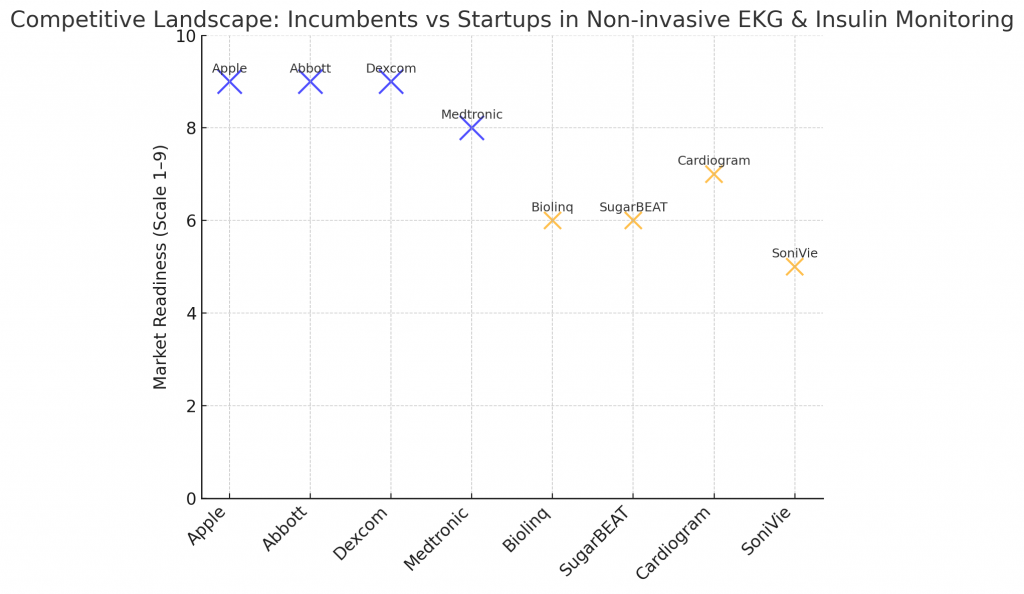
Large and experienced players are bringing scale and regulatory expertise:
- Apple — consumer wearables with ECG features and ongoing work in glucose sensing.
- Dexcom & Abbott — category leaders in continuous glucose monitoring, expanding sensing approaches.
- Medtronic & Omron — clinical-grade wearables and remote monitoring integrations.
- AliveCor & iRhythm — portable EKG devices, diagnostic workflows, and long-duration patches.
- Biolinq, SugarBEAT, SoniVie, Glucotrack — startups focused on patch-based or ultrasound/optical glucose sensing.
Startups often drive sensing innovation; incumbents add distribution, clinical trials and reimbursement muscle.
What patents and research trends should you watch for?
Patent signals to track:
- Hybrid sensing stacks combining optical + impedance + electrochemical sensors.
- Calibration approaches addressing skin pigmentation, hydration, and temperature.
- Motion artifact reduction and real-world filtering algorithms.
- Data-security and interoperability claims for cloud/edge data transfer.
A focused patent landscape helps spot white-space, avoid infringement, and pick licensing targets.
How mature is non-invasive EKG and insulin monitoring (TRL and timeline)?
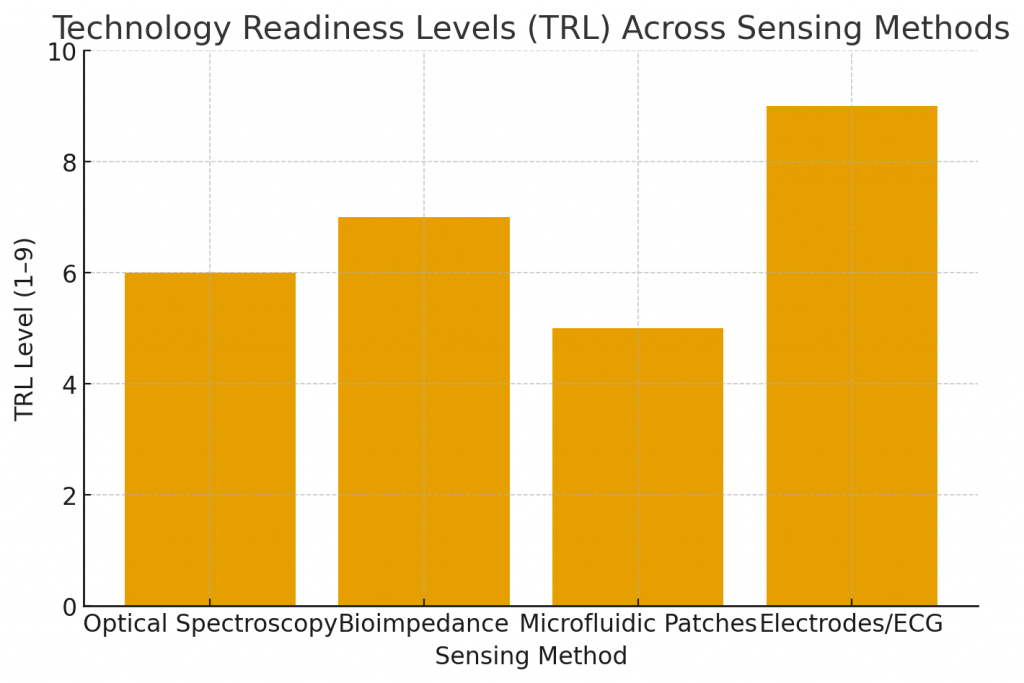
- EKG capabilities in consumer wearables (single-lead rhythm, AFib screening) are TRL 8–9—market-proven in many regions.
- Truly non-invasive glucose estimation (optical/molecular sensing) varies: many methods sit at TRL 5–7 (prototype to pilot). Minimally invasive CGMs are further ahead.
- Timeline: expect steady improvements in accuracy and regulatory progress over the next 2–5 years for several non-invasive approaches; full integration into mainstream chronic-care workflows will follow clinical validation and payer alignment.
What are the main clinical and consumer applications?
Primary use cases:
- Diabetes self-management: continuous trend insight, early hypo/hyper alerts, remote insulin titration support.
- Cardiac screening and monitoring: AFib detection, arrhythmia follow-up, post-procedural surveillance.
- Remote patient monitoring: feeding telemedicine platforms and care-management programs.
- Preventive care & fitness: early screening, lifestyle coaching, integration with wellness programs.
- Elder care: passive monitoring with caregiver alerts.
These applications reduce friction for patients while enabling proactive clinical interventions.
What regulatory and standards challenges exist?
Critical requirements and frameworks:
- ISO 13485 (quality management) and ISO 14971 (risk management).
- IEC 60601 series for electro-medical safety and specific ECG device standards.
- ISO/IEEE 11073 for device communication and interoperability.
- National regulatory pathways (FDA, CE/MDR, CDSCO) require clinical evidence—especially for devices that guide therapy (e.g., insulin dosing).
Accuracy, repeatability, explainability of AI models, and data protection (HIPAA/GDPR) are recurring evaluation points.
How can companies overcome common technical hurdles?
Practical mitigations:
- Use hybrid sensing architectures to hedge against single-sensor weaknesses.
- Build personalized calibration routines that adapt to skin tone and physiology.
- Design clinical trials that reflect real-world activity (motion, temperature, sweat).
- Prioritize battery efficiency, intuitive UX, and seamless clinician integration to boost adoption.
What high-value opportunities does non-invasive EKG and insulin monitoring create?
New value pools:
- Bundled hardware + subscription analytics for patients and providers.
- AI-driven predictive alerts that prevent hospitalizations.
- Clinical trial acceleration through continuous, real-world endpoints.
- Licensing of sensing or calibration algorithms to device OEMs.
The richest opportunities sit where clinical value, regulatory clarity, and payer economics align.
How will this technology change current care pathways?
Expected shifts:
- Move from episodic testing to continuous, context-rich monitoring.
- Fewer emergency admissions due to earlier intervention.
- Decentralized care: home titrations, virtual consults, and remote therapy adjustments.
- Data-driven population health programs with better preventive reach.
How can PatentsKart help teams working on non-invasive EKG and insulin monitoring?
PatentsKart’s support areas:
- Patent landscaping to identify freedom-to-operate and white-space opportunities.
- Competitive intelligence on incumbents and startup filings.
- Prior art searches and patent drafting to strengthen IP portfolios.
- Market, regulatory and standards mapping to align R&D with compliance needs.
- Clinical strategy advisory to design validation plans that support both regulatory and IP objectives.
Why does this matter now?
Non-invasive EKG and insulin monitoring are converging technologies that solve long-standing barriers in chronic care: comfort, continuity, and context. When sensor hardware, AI, and clinical validation come together, patients gain autonomy and clinicians gain continuous insights—creating a more proactive and efficient healthcare system. By embedding non-invasive EKG and insulin monitoring into everyday devices and clinical pathways, teams can accelerate prevention, personalize therapy, and reduce acute events.
Frequently Asked Questions
1. What are the most accurate non-invasive glucose monitoring methods today?
Accuracy varies: current leaders combine optical spectroscopy with minimally invasive CGM comparisons. Hybrid methods (optical + microfluidic sampling) show promising accuracy in trials but need broader validation.
2. Can a smartwatch replace a medical EKG for diagnosis?
Smartwatches are useful screening tools and can capture single-lead ECGs. However, full diagnosis often requires multi-lead ECGs and clinician interpretation—wearables augment, not entirely replace, clinical ECGs.
3. How soon will non-invasive glucose monitors be widely available?
Several vendors and startups project broader availability within 2–5 years, contingent on clinical validation, regulatory approvals, and payer acceptance.
4. Are non-invasive monitors safe and reliable for elderly users?
When clinically validated and designed for low-maintenance use, these devices are safe. Usability, reliable alerts, and battery life are critical for elderly adoption.
5. How should startups protect their technology in this space?
Focus on patenting core sensing methods, calibration algorithms, and user-workflow innovations. Combine IP strategy with clinical evidence and regulatory planning to build defensibility.







