Introduction
For decades, drug delivery has been dominated by syringes and hypodermic needles—an approach that, while effective, has several inherent problems: patient discomfort, risk of infections, needlestick injuries, poor compliance, and medical waste. In a world that is increasingly prioritizing patient-centric care, technological efficiency, and public health safety, the traditional needle is becoming outdated.
Needle-free injection technologies are emerging not as an alternative—but as the inevitable future of drug delivery. From microneedle patches to jet injectors and transdermal systems, these innovations are transforming both healthcare delivery and the pharmaceutical value chain. The implications for investors, pharmaceutical companies, medical device manufacturers, and IP stakeholders are profound.
What Is a Needle-Free Injection?
Needle-free injection refers to the administration of drugs without piercing the skin with a conventional needle. These systems are designed to improve patient compliance, reduce infection risk, and simplify drug administration.
Major Categories:
Jet Injectors: Deliver liquid medication using high-pressure streams.
Microneedle Arrays: Tiny, minimally invasive needles embedded in patches.
Transdermal Patches: Passive or active drug release through the skin.
Inhalable Delivery Systems: Particularly for insulin and vaccines.
Electroporation and Sonophoresis: Energy-assisted delivery systems for targeted applications.
| Type | Key Advantage | Primary Use |
|---|---|---|
| Jet Injector | Fast, deep penetration | Vaccines, biologics |
| Microneedle Patch | Painless, self-administered | Hormones, insulin, vaccines |
| Transdermal Patch | Sustained release | Pain meds, nicotine |
| Inhalable System | Non-invasive, fast absorption | Insulin, peptides |
Why Now? Market Drivers Accelerating Needle-Free Technologies
Several converging trends are making needle-free injection systems not just desirable, but necessary.
1. Rising Demand for Painless Drug Delivery
Needle phobia affects a significant portion of the population, especially children and the elderly. Pain-free alternatives improve compliance, especially for chronic diseases requiring long-term administration.
2. Explosion of Biologics and Complex Therapies
Biologics often require subcutaneous or intramuscular delivery—needle-free systems like jet injectors and microneedle arrays are better suited for these large-molecule drugs.
3. Post-COVID Public Health Awareness
Mass vaccination campaigns exposed the limits of traditional syringes. Needle-free systems can scale more efficiently and reduce biomedical waste.
4. Healthcare Access and Self-Administration
With global interest in remote care and self-administered therapies, these systems enable safe, accurate, and home-based drug delivery.
5. Global Regulatory Push
WHO, FDA, and EMA have acknowledged the value of microneedle and jet-injection systems in improving healthcare access and compliance.
Patent and Innovation Landscape
Leading Innovators
- Portal Instruments (USA) – high-pressure, computer-controlled jet injectors.
- PharmaJet – FDA-cleared needle-free injectors used for polio vaccination programs.
- 3M Drug Delivery Systems – microneedle patches for vaccines and insulin.
- Zogenix – patented systems for CNS disorder drug delivery.
- Micron Biomedical – focused on pediatric vaccine delivery in LMICs.
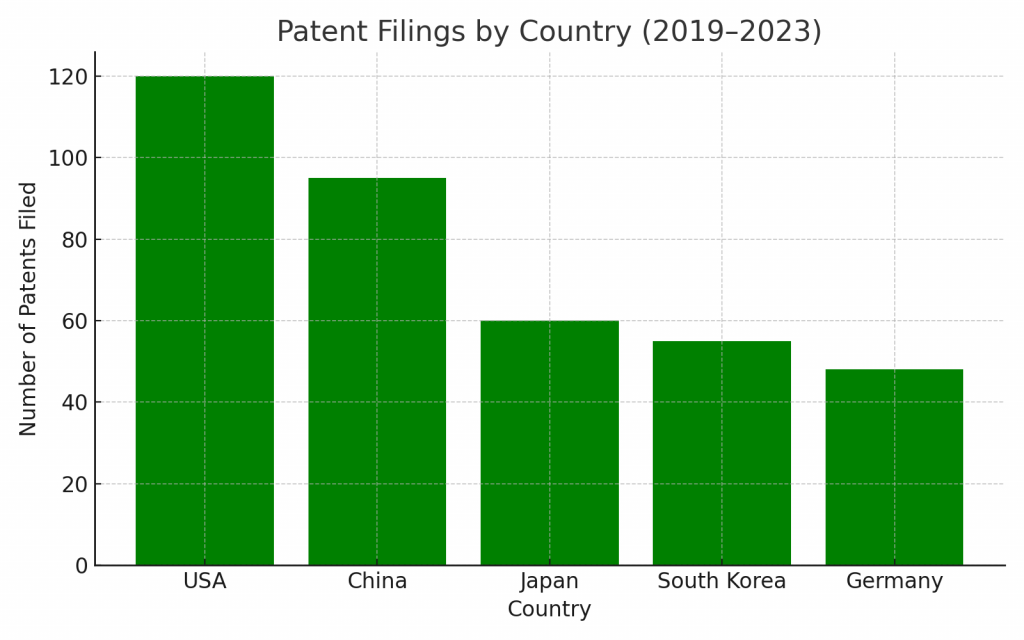
Patent Trends
The past five years have seen a 40%+ increase in global filings related to needle-free drug delivery. The majority of filings are concentrated in:
- United States
- China
- Japan
- South Korea
- Germany
| Company | Patent Focus | Status |
|---|---|---|
| Portal Instruments | Jet pressure control | Granted (US) |
| PharmaJet | Disposable syringe modules | Granted (Global) |
| Micron Biomedical | Vaccine microneedles | Pending (India, US) |
Key Applications of Needle-Free Injection Technology
1. Vaccinations
- Mass immunization campaigns for polio, influenza, and COVID-19
- Key benefit: reduced needlestick injuries, faster deployment
2. Insulin Delivery
- Especially critical in markets with rising Type 1 and 2 diabetes
- Promotes better glycemic control through improved adherence
3. Hormone Therapy
- Hormone replacement and fertility treatments benefit from microneedle and transdermal systems
4. Cancer Therapies
- Needle-free systems are being trialed for targeted biologic delivery
5. Emergency Medicine
- Jet injectors can deliver medications like epinephrine instantly in field settings
Challenges Slowing Adoption
Despite technical advancements, certain friction points remain.
- Cost and Manufacturing Complexity: Device production is more intricate than standard syringes.
- Regulatory Disparity: Standards vary by country, delaying global commercialization.
- Cold Chain Sensitivity: Some needle-free systems still require temperature-controlled storage.
- Market Education: Clinician and patient awareness is still limited in developing economies.
Emerging Standards and Global Regulations
| Region | Standard/Agency | Focus Area |
|---|---|---|
| USA | FDA, ISO/IEC 21966 | Jet injectors, safety mechanisms |
| EU | CE Mark, EMA | Microneedle patches |
| India | CDSCO | Experimental regulatory inclusion |
| WHO | PQS (Prequalified Devices) | Vaccine delivery in global campaigns |
There is a growing movement toward establishing unified ISO standards for device safety, efficacy, and dosage consistency.
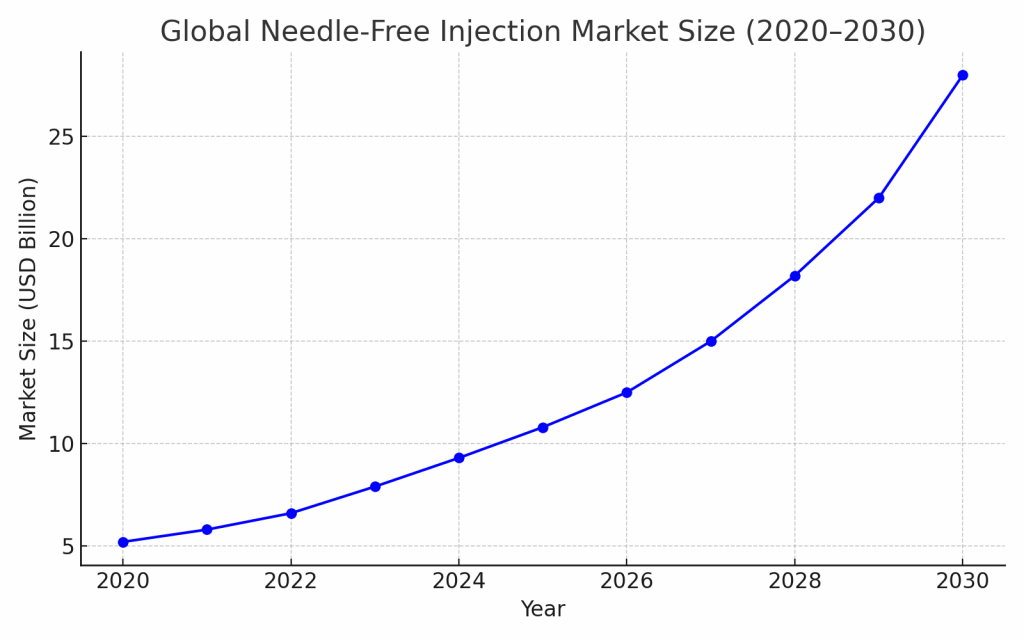
Market Outlook and Investment Trends
The global needle-free injection market is projected to grow from USD 12.5 billion in 2024 to over USD 28 billion by 2030, at a CAGR of 15–18%.
Active Investment Areas:
- Startups in controlled jet injection tech
- Licensing of microneedle IP by pharma companies
- Partnerships between biotech and device OEMs
M&A Activity:
- Recent acquisition of microneedle tech startups by Johnson & Johnson and Pfizer indicates strong consolidation interest.
Strategic Opportunities for Stakeholders
For Investors:
- Focus on startups with FDA/CE-pending needle-free platforms
- Prioritize IP-rich firms with strong licensing potential
- Track academic spinouts in microneedle and electroporation delivery
For Pharmaceutical Companies:
- Explore co-development models with delivery device companies
- Adapt existing biologics for needle-free formats
- License or acquire patented technologies for regional scale-up
For Policymakers and Regulators:
- Fast-track approvals with defined pathways
- Support pilot studies in public vaccination programs
- Incentivize local manufacturing of reusable systems
Needle-Free Injection in India
India represents a vast, under-tapped market for needle-free technologies:
- High burden of diabetes, TB, hepatitis, and other chronic illnesses
- Strong domestic pharma base to support co-development
- Government-backed vaccination programs open for tech trials
Current Gaps:
- Very limited domestic innovation in microneedle design
- Lack of formal regulatory guidance from CDSCO
- Low clinician awareness in tier-2 and tier-3 cities
Actionable Strategy: Build India-specific IP around indigenous microneedle tech with licensing potential for SE Asia and Africa.
How PatentsKart Can Help
PatentsKart enables stakeholders in the needle-free injection ecosystem to make informed, strategic decisions by providing:
- Patent Landscape Reports across global jurisdictions
- Technology Scouting for early-stage innovations
- White Space Mapping to identify unmet opportunities
- Competitor Analysis of filing trends and M&A targets
- IP Due Diligence for investor-grade insights
Whether you’re an investor looking to bet on the right startup or a pharmaceutical company planning product reformulation, PatentsKart delivers the IP intelligence you need to move decisively.
Frequently Asked Questions(FAQ)
-
What is a needle-free injection and how does it work?
A needle-free injection delivers medication without a traditional needle, using jet pressure, microneedles, or skin-absorption technologies.
-
Are needle-free injections safe and effective?
Yes. When approved by regulators like the FDA or WHO, these systems are proven to be safe, accurate, and effective for vaccines, insulin, and more.
-
What are the advantages of needle-free injections over needles?
They reduce pain, eliminate needlestick injuries, lower medical waste, and improve patient compliance—especially for children and chronic patients.
-
Who are the top companies developing needle-free injection technology?
Leading names include Portal Instruments, PharmaJet, 3M, Micron Biomedical, and Zogenix, with growing innovation in Asia and India.
-
Can I get vaccines or insulin through needle-free injections in India or the US?
Yes, they are available in the US and being introduced in India through pilot programs, especially in diabetes care and vaccination drives.
Conclusion
Needle-free injections are no longer an R&D curiosity—they are a validated, scaling solution to several of the most pressing challenges in healthcare delivery. From improving patient comfort to enabling large-scale immunization, the value proposition is overwhelming. As technology matures and regulatory frameworks evolve, those who move early—whether through investment, partnership, or IP acquisition—will hold critical advantages in a rapidly transforming sector.
To explore deeper insights into breakthrough healthcare technologies, visit www.patentskart.com or contact us at info@patentskart.com.







